Chapter 1 Chemical Reactions and Equations
CBSE Notes for Class 10 Science Chemistry
➣ Physical Change: Change in physical properties.
– Melting
– Boiling
– Condensation
– [Note- No change occurs in the identity of the substance].
➣ Chemical Change:
– Atoms in the reactants are rearranged to form one or more different substances.
– Old bonds are broken, new bonds are formed.
– Reactants lose their properties to form product of different properties.
4 Fe(s) + 3O2 →2Fe2O3 (rust). Iron Oxygen Ferric oxide
➣ Chemical equation:
The symbolic representation of a chemical reaction is called a
chemical equation.
Features of a chemical equation:
• The reactants are written on the left hand side with a plus sign between them.
• The products are written on the right hand side with a plus sign between them.
• An arrow separates the reactants from the products. The arrow head points towards the products and indicates the direction of the reaction.
Skeletal chemical equation: A chemical equation which simply represents the symbols and formulas of reactants and products taking part in the reaction is known as skeletal chemical equation for a reaction.
For example: For the burning of Magnesium in the air, Mg + O2 → MgO is the skeletal equation.
• Balanced chemical equation: A balanced equation is a chemical equation in which number of atoms of each element is equal on both sides of the equation i.e number ofatoms of an element on reactant side = number of atoms of that element on the product side.
➣ Identification:
| Combustion : | AB + O2 → Oxide of A & B. |
| Combination : | A + B → C |
| Decomposition : | AB → A + B |
| Displacement : | A + BC (aq) → AC (aq) + B |
| Double Displacement : | AB (aq) + CD (aq) → AD (aq) + CB |
Definitions with examples :
1. Combination Reaction :Two or more reactant combine to form a single product.
2 Mg (s) + O2 (g) → 2 MgO Magnesium Oxygen Magnesium oxide (White ash)
(basic) turns Red litmus blue
2. Decomposition Reaction: A single compound decomposes or break down to give two or moresimpler substances.
2 FeSO4 (s).![]() Fe2 O3 (s) + SO2 (g) + SO3(g) Ferrous Sulphate Ferric oxide Sulphur Sulphur (green) (brown) dioxide trioxide
Fe2 O3 (s) + SO2 (g) + SO3(g) Ferrous Sulphate Ferric oxide Sulphur Sulphur (green) (brown) dioxide trioxide
3. Displacement Reaction: A more reactive element [metal] displaces less reactive elemen [metal] from its aqueous salt soln Fe (s) + ZnSO4 (aq) → FeSO4 (aq) + Zn (s) (Colourless) (green)
4. Double Displacement Reaction: Aqueous soln of two ionic compounds react by exchange of their ions is called double displacement Reaction BaCl2 (aq) + Na2SO4 (aq) → BaSO4 (s) + 2 NaCl (aq) Pb(NO3)2 + 2 KI (aq) → PbI2 ( ↓ ) + 2 KNO3 (aq)
5. Oxidation Reaction: In oxidation reaction, addition of oxygen or removal of hydrogen or loss of electron takes place. 2 Mg(s) + O2 (g) → 2MgO (s) 2 Cu + O2 → 2 CuO (Black ) (Copper II Oxide)
6. Reduction Reaction: In reduction Reaction addition of hydrogen or removal of oxygen or gain of electron takes place. CuO(s) + H2 (g) →Cu(s) + H2 O (l)
7. Redox Reaction: Reaction involving both oxidation and reduction simultaneously CuO (s)+ H2 → Cu(s) + H2 O (l).
8. Exothermic Reaction: Reaction in which heat is evolved. C (s) + O2 (g) → CO2 (g) + Heat
10. Neutralisation Reaction : When an acid and a base react together to form salt and water. HCl (aq) + NaOH (aq) → H2O (l) + NaCl (aq) (acid) (base) (Water) (Salt) Hydrochloric acid Sodium hydroxide Sodium Chloride
Law of Conservation of Mass
In a chemical reaction matter is conserved. Total no. of atoms = Total no. of atoms Total mass = Total mass. [While Balancing a Chemical Equation Formula of reactants and products should not be changed]. Balancing:
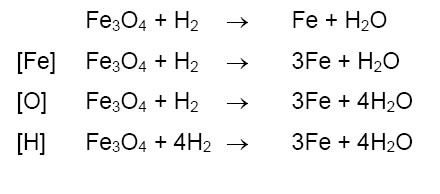
➣ Corrosion: Process of slowly reacting up of metals due to attack of atmospheric gases like O2 ,CO2M etc.
![]()
Rust(hydrated Iron (III)oxide)
Prevention:Painting, Galvanization, oiling greasing.
Corrosion of Aluminium has advantage , since Al2O3 formed as a result
of corrosion act as protective layer.
➣ Rancidity:
Oxidation of oils or fats in a flood, resulting into a bad smell and taste.
Prevention: Adding anti-oxidants.
Vacuum Packing
Replacing air by Nitrogen
Refrigeration of food stuf
➣ [ KEY POINTS ]
• A chemical reaction involves a chemical change in which substances react to form new substances with entirely new properties. Substances that react or take part in the reaction are known as reactants and the ubstances formed are known as products.
• During a chemical reaction, there is a breaking of bonds between atoms of the reacting molecules to give products.
• A chemical reaction can be observed with the help of any of the following observations:Evolution of a gas
1.Change in temperature
2.Formation of a precipitate
3.Change in colour
4.Change of state
• Physical change: If a change involves change in colour or state but no new substance is formed, then it is a physical change.
• Chemical change: If a change involves formation of new substances, it is a chemical change.
• Exothermic and endothermic reactions: If heat is evolved during a reaction, then such a reaction is known as Exothermic reaction. If heat is absorbed from the surroundings, then such a reaction is known as endothermic reaction.
• As per the law of conservation of mass, the total mass of the elements present in the products of a chemical reaction is equal to the total mass of the elements present in the reactants.
• The process of equating the number of atoms on both the sides of a chemical equation is known as balancing of a chemical equation.
• The first step in balancing a chemical equation is to write the number of atoms of each element present on the left hand side and right hand side.
• We should always start balancing with the compound that contains maximum number of atoms. It can be reactant or a product. Then in that compound select the element which has the maximum number of atoms.
• While balancing a chemical equation, the molecular formulas of the reactants and products should not change. The molecular formulas are simply multiplied by suitable coefficients.
• To make a chemical equation more informative, the reaction conditions such as temperature, pressure or catalyst are written on the arrow separating the reactants and products.
• The evolution of gas is indicated by an upward arrow.
• The formation of precipitate is indicated by a downward arrow.
• Heat evolved during the reaction is written as + Heat on the product side.
• Heat absorbed during the reaction is written as + Heat on the reactant side.
• Combination reaction is a reaction in which 2 or more substances combine to give a single product.
• Combination reaction can be between two elements, between an element and a compound or between two compounds.
• Decomposition reaction: In a decomposition reaction, a single reactant decomposes to give two or more products.
• Decomposition reactions require energy in the form of heat, light or electricity
Types of decomposition reactions:
• Decomposition reactions which require heat are known as thermolytic.
• Decomposition reactions which require light are known as photolytic.
• Decomposition reactions which require electricity are known as electrolytic.
• Displacement reaction: A reaction in which a more active element displacesless active element from its salt solution.
• The reactivity series is a list of metals arranged in the order of decreasing reactivity.
• The most reactive metal is placed at the top and the least reactive metal is placed at the bottom.
• Double displacement reaction: A chemical reaction in which there is an exchange of ions between the reactants to give new substances is called double displacement reaction.
• Precipitation reaction: An insoluble solid known as precipitate is formed during a double displacement reaction. Such reactions are also known as precipitation reactions.
• Redox reaction: A reaction in which oxidation and reduction take place simultaneously in a reaction, is known as a redox reaction.
• Oxidation is a chemical process in which a substance gains oxygen or loses hydrogen.
• Reduction is a chemical process in which a substance gains hydrogen or loses oxygen.
• If a substance gains oxygen or loses hydrogen during a reaction, it is said to be oxidised.
• If a substance gains hydrogen or loses oxygen during a reaction, it is said to be reduced.
• A substance that loses oxygen or gains hydrogen is known as an oxidising agent.
• A substance that loses hydrogen or gains oxygen is known as a reducing agent.
• An oxidising agent gets reduced whereas a reducing agent gets oxidised.
• In terms of electronic concept, Oxidation is defined as a loss of electrons while reduction is defined as a gain of electrons.
• Corrosion is the slow eating up of metals by the action of air and moisture on their surfaces. Corrosion in case of Iron is known as Rusting.
• Chemically, rust is hydrated ferric oxide (Fe2O3.xH2O)
• Advantages of corrosion: Though corrosion is undesirable, it can be advantageous in case of aluminium which on exposure to air, gets coated with a protective layer of aluminium oxide. This protects the metal nderneath from further corrosion and damage.
• Rancidity: When oils and fats or foods containing oils and fats are exposed to air, they get oxidised due to which the food becomes stale and gives a bad aste or smell. This is called Rancidity.
• Rancidity can be prevented by: a Adding antioxidants i.e. the substances which prevent oxidation b Refrigeration c Storing the food in air-tight containers
SUGGESTED ACTIVITIES
• Combination reaction b/w Magnesium ribbon and Oxygen from air.
• Combination reaction b/w Quick Lime and Water.
• Thermal decomposition of Ferrous Sulphate.
• Thermal decomposition of Lead Nitrate
• Formation of hydrogen gas by the action of dil. Sulphuric Acid on Zinc
• Displacement reaction b/w Iron /Copper Sulphate,Zinc/ Copper Sulphate
• Double displacement reaction b/w Lead Nitrate & Potassium Iodide.
• Double displacement reaction b/w Sodium Sulphate & Barium Chloride.
• Photodecomposition of Silver Chloride
• Oxidation of Copper to Copper Oxide
• Exothermic and Endothermic Reaction.
QUESTION BANK
Very Short answer type questions :
1. What happens when magnesium ribbon burns in air?
Ans. When magnesium ribbon burns in air, it combines with the oxygen to form magnesium oxide. 2Mg(s) + O2(g) → 2MgO(s)
2. Name the gas evolved when zinc reacts with dil. HCl.
Ans. Hydrogen gas is evolved.
3. What is a chemical equation?
Ans. A chemical equation is a symbolic notation that uses formulae instead of words to represent
a chemical equation.
4. On what chemical law, balancing of chemical equation is based?
Ans. Balancing of a chemical equation is based on the law of conservation of mass.
5. Represent decomposition of ferrous sulphate with the help of balanced chemical
equation.
Ans. 2FeSO4(s) → Fe2O3(s) + SO2(g) + SO3(g)
6. When carbon dioxide is passed through lime water, it turns milky, why?
Ans. Lime water (calcium hydroxide) combines with carbon dioxide to form a suspension
of calcium carbonate which makes lime water milky.
Ca(OH)2(aq) + CO2(g) → CaCO3(s) + H2O(l)
7. A zinc rod is left for nearly 20 minutes in a copper sulphate solution. What change
would you observe in zinc rod?
Ans. Zinc rod will change into zinc sulphate.
8. What type of reaction is this: Na2SO4 + BaCl2 → BaSO4 +2NaCl
Ans. It is a double displacement reaction.
9. Identify the compound oxidized in the following reaction. H2S (g) + Cl2 → S(s) + 2HCl (g)
Ans. H2S is oxidized.
10. What is rust?
Ans. Rust is mainly hydrated iron (III) oxide, Fe2O3→xH2O.
Short answer type questions:
1. An iron knife kept dipped in a blue copper sulphate solution turns the blue solution light green. Why?
Ans. As we know iron is more reactive than copper. So, it displaces Cu from CuSO4 solution and forms ferrous sulphate which is of Light Green Colour. CuSO4 (aq) + Fe (s)→ FeSO4 (aq) + Cu(s) Blue colour light green colour
2. A copper coin is kept in a solution of silver nitrate for some time. What will happen to the coin and the colour of the solution?
Ans: We know that copper is more reactive than silver, so it will displace silver from its salt solution: Cu(s) + 2AgNO3(aq) →Cu(NO3)2(aq)+2Ag(s) So the solution will turn blue due to the formation of copper nitrate.
3. What do you understand by precipitation reaction? Explain with suitable examples.
Ans. Precipitate. When two reactants react and product formed remains insoluble and settles as a solid it is called a precipitate. Such reactions in which precipitate is formed are called precipitation reactions.
For example,
i) When aqueous solution of sodium sulphate is mixed with the aqueous solution or barium chloride, barium sulphate comes in the form of white precipitate
Na2SO4 (aq)+BaCl2(aq) → BaSO4(↓)+2NaCl(aq)
11 ZIET CHANDIGARH
ii) When aqueous solution of sodium chloride is mixed with the aqueous solution of silver nitrate, silver chloride comes in the form of white precipitate.
4. What is lime-water test for the detection of carbon dioxide?
Ans. When carbon dioxide gas is passed through lime water, it turns milky due to the formation of milky suspension (precipitate) of calcium carbonate. Carbon dioxide is produced by the action of dilute HCl on sodium carbonate. Na2CO3(s)+2HCl(aq)→2NaCl+H2O(l)+CO2
Carbon dioxide gas produced in this reaction is passed through lime water it changes to milky colour due to the formation of calcium carbonate.
Long answer type questions:
1. What is corrosion? State the conditions necessary for rusting of iron. How rusting is harmful ?
Ans: Corrosion: The process of eating away of the metal by the action of atmospheric reagents changing the metal into its compound is called corrosion.
Rusting of Iron : When iron and iron objects are exposed to atmosphere, they are attacked by air and moisture (water) of the atmosphere and a brown and orange colored layer is formed on the surface. It is called rust which is mainly hydrated iron (III) oxide Fe2O3→xH2O.
Harmful Effect of Rusting : Hydrated iron (III) oxide is brittle substance and moves away from the surface thus the object is damaged. The objects get holes, cavities and rough surface. Conditions necessary for rusting:
i) Open surfaces of the metal.
ii) Presence of air (Oxygen).
iii) Presence of moisture (water).
2. What is rancidity? Write the common method to prevent it.
Ans. When food item are kept unprotected for some time, they give some unpleasant smell and taste and become rancid.
This process is called rancidity. Actually, the micro organisms oxidize the fat and oils present in them. So,oxidation of food items need to be prevented to protect them.Common methods to Prevent Rancidity of Food item
i) Keeping the food at low temperature
ii) Keeping food item in air tight containers
iii) By filling nitrogen in the food storage bags
3. a) Why cannot a chemical change be normally reversed ?
b) Why is it always essential to balance a chemical equation?
c) What happens when CO2 gas is passed through lime water and why does it
disappear on passing excess CO2?
d) Can rusting of iron takes place in distilled water?
Ans: a. In a chemical change some bonds are broken and some bonds are formed. The products are quite different from the reactants. Therefore it normally can’t be reversed.
b. A chemical equation has to be balanced to satisfy the law of conservation of mass.
c. On passing CO2 gas through lime water, it turns milky due to formation of
insoluble calcium carbonate which dissolves on passing excess CO2 due to
formation of soluble calcium bicarbonate.
Ca(OH)2 + CO2(g) → CaCO3(s) + H2O(l)
CaCO3(s) + H2O(l) + CO2 (g) → Ca(HCO3)2(soluble)
d. No, rusting of iron cannot take place in distilled water because it neither contains dissolved oxygen nor CO2 both are essential for rusting of iron.
1. The marble statues often slowly get corroded when kept in open for a long time.
Assign a suitable explanation
Ans. SO2* NO2 gases are released into the atmosphere from various sources. These dissolve in rain water to give acid which corrodes marble statue
2SO2 + O2→ 2SO3 ; H2O+SO3 →H2SO4
2NO2 + H2O→2HNO3
CaCO3 + H2SO4→CaSO4 +H2O + CO2
CaCO3+2HNO3 → Ca(NO3)2+H2O+CO2
2. You are given the following materials (a) marble chips (b)dilute hydrochloric acid (c) Zinc granules ,identify the type of reaction when marble chips and Zinc granules are added separately to acid taken in two test tubes .
Ans. (a) marble chips react with dilute hydrochloric acid to form calcium chloride and carbon dioxide .it is a double displacement reaction
CaCO3+2HCl → CaCl2 + H2O +CO2
(b) Zinc granules react with dilute hydrochloric acid to give hydrogen gas. it is a displacement reaction Zn(s)+2HCl → ZnCl2(aq)+H2((g)
3. The gases hydrogen & chlorine do not react with each other even if kept together for a long time . However, in the presence of sunlight, they readily combine. What does actually happen?
Ans. In Chemical reactions, energy is needed to break the bonds present in the reacting molecules so that they may combine to form the products. In this reaction, sunlight is the source of energy in the form of photons. The energy made available by sunlight helps in breaking the bonds & this leads to chemical reaction between hydrogen& chlorine. H2(g) + Cl2(g) sunlight →2HCl (g)
4. A, B and C are three elements which undergo chemical reactions in the following way:
A2O3 + 2B→B2O + 2A
3 CSO4 + 2B → B2(SO4)3 + 3C
3 CO+ 2A→ A2O3 +3C
Answer the following
a) Which element is most reactive?
b) Which element is least reactive ?
Ans: a) The most reactive element is ‘B’. It has displaced both ‘A’ and ‘c’ from their
compounds.
b) The least reactive element is ‘C’ as it has been displaced by both ‘A’ and ‘B’ .
5. A water insoluble substance =X‘ on reacting with dilute H2SO4 released a colourless and odourless gas accompanied by brisk effervescence. When the gas was passedthrough water, the solution obtained turn blue litmus red . On bubbling the gas through lime water, it initially became milky and the milkiness disappeared when the gas was passed in excess. Identify the substance =X‘ and write the chemical equations of the reaction involved .
6. Ahmad took a magnesium ribbon (cleaned) and burned it on a flame. The white powder formed was taken in a test tube and water was added to it. He then tested the solution formed with red and blue litmus paper. What change was seen? Why?
Ans. Red litmus paper turned blue. Blue litmus paper remained blue. This is because the magnesium ribbon on burning in air forms the white magnesium oxide. Which dissolved in water, it forms magnesium hydroxide, which is basic in nature
7. Give one example of a combination reaction in which an element combines with a compound to give you a new compound.
Ans. O2 + 2SO2 → 2SO3 8NH3 + 3Cl2→ 6NH4Cl
Reaction worksheet
Write balanced equations for the following word equations.
1. Potassium chloride + Silver nitrate→Potassium nitrate + Silver chloride
2. Aluminum hydroxide + Sodium nitrate→ Aluminum nitrate + Sodium hydroxide
3. Iron metal + Copper(II) sulphate → Iron(ii) sulphate + copper metal
4. Aluminum metal + Copper(II) chloride →Aluminum chloride + copper metal
5. Potassium bromide→Potassium metal + Bromine
6. Calcium carbonate →Calcium oxide + Carbon dioxide gas
7. Zinc metal + Oxygen gas → Zinc oxide
8. Chlorine gas + Sodium metal →Sodium chloride
9. Aluminum sulphate + Barium chloride→Aluminum chloride + Barium sulphate
10. Sodium hydrogen carbonate → Sodium carbonate + Carbon dioxide + Water












Nice
ReplyDelete